Posts
Article of the month: Exercise‐induced attenuation of treatment side‐effects in patients with newly diagnosed PCa beginning androgen‐deprivation therapy: a randomised controlled trial
Every month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urology community, a video prepared by the authors and a visual abstract providing a graphical representation of the article; we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, we recommend this one.
Exercise‐induced attenuation of treatment side‐effects in patients with newly diagnosed prostate cancer beginning androgen‐deprivation therapy: a randomised controlled trial
Wilphard Ndjavera*, Samuel T. Orange†, Alasdair F. O’Doherty†, Anthony S. Leicht‡, Mark Rochester*, Robert Mills* and John M. Saxton†§
*Department of Urology, Norfolk and Norwich University Hospital, Norwich, UK, †Department of Sport, Exercise and Rehabilitation, Faculty of Health and Life Sciences, Northumbria University, Newcastle upon Tyne, UK, ‡Sport and Exercise Science, College of Healthcare Sciences, James Cook University, Townsville, QLD, Australia and §Norwich Medical School, Faculty of Medicine and Health Sciences, Norwich Research Park, University of East Anglia, Norwich, UK
Abstract
Objectives
(i) To assess whether exercise training attenuates the adverse effects of treatment in patients with newly diagnosed prostate cancer beginning androgen‐deprivation therapy (ADT), and (ii) to examine whether exercise‐induced improvements are sustained after the withdrawal of supervised exercise.
Patients and Methods
In all, 50 patients with prostate cancer scheduled for ADT were randomised to an exercise group (n = 24) or a control group (n = 26). The exercise group completed 3 months of supervised aerobic and resistance exercise training (twice a week for 60 min), followed by 3 months of self‐directed exercise. Outcomes were assessed at baseline, 3‐ and 6‐months. The primary outcome was difference in fat mass at 3‐months. Secondary outcomes included: fat‐free mass, cardiopulmonary exercise testing variables, QRISK®2 (ClinRisk Ltd, Leeds, UK) score, anthropometry, blood‐borne biomarkers, fatigue, and quality of life (QoL).
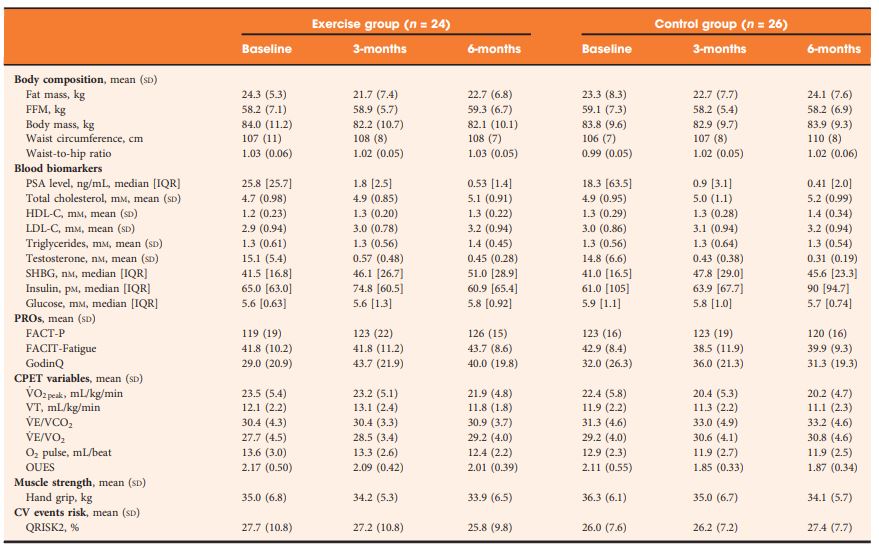 Table 2 Outcomes at baseline, 3- and 6-months.
Table 2 Outcomes at baseline, 3- and 6-months.
Results
At 3‐months, exercise training prevented adverse changes in peak O2 uptake (1.9 mL/kg/min, P = 0.038), ventilatory threshold (1.7 mL/kg/min, P = 0.013), O2 uptake efficiency slope (0.21, P = 0.005), and fatigue (between‐group difference in Functional Assessment of Chronic Illness Therapy‐Fatigue score of 4.5 points, P = 0.024) compared with controls. After the supervised exercise was withdrawn, the differences in cardiopulmonary fitness and fatigue were not sustained, but the exercise group showed significantly better QoL (Functional Assessment of Cancer Therapy‐Prostate difference of 8.5 points, P = 0.034) and a reduced QRISK2 score (−2.9%, P = 0.041) compared to controls.
Conclusion
A short‐term programme of supervised exercise in patients with prostate cancer beginning ADT results in sustained improvements in QoL and cardiovascular events risk profile.
IP4-CHRONOS is launched
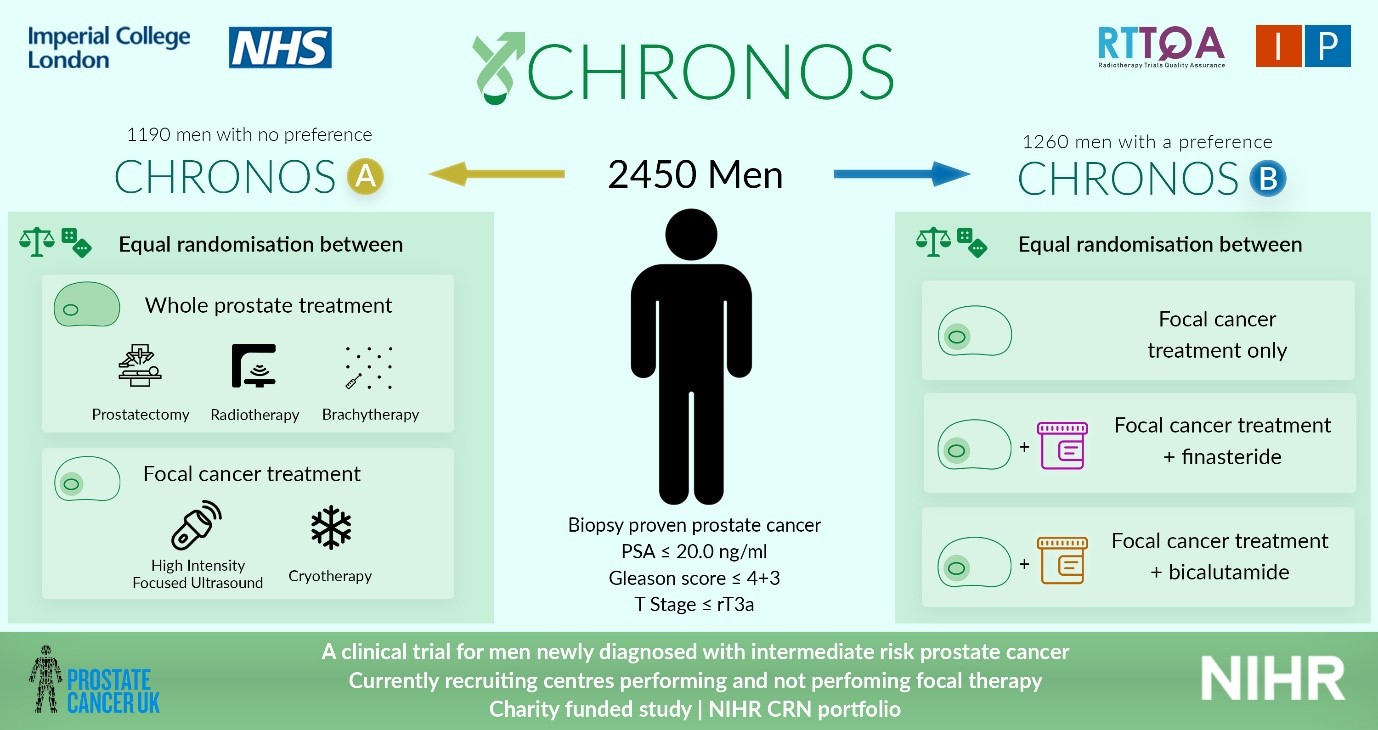
IP4- CHRONOS is open! CHRONOS is a phase II randomised control trial, that will review the outcomes (including oncological, functional, quality of life and cost-effectiveness) of focal therapy against those from radical therapy, in men with newly diagnosed localised clinically significant prostate cancer.
All men newly diagnosed with low-intermediate risk prostate cancer, confined to the prostate, with a life expectancy of at least 10 years will be screened for eligibility. Men must be well enough to undergo the interventions outlined in the trial prior to being enrolled.
Men will then have a choice of enrolling into CHRONOS A or CHRONOS B. CHRONOS A will randomise men to having radical whole gland treatment (radiotherapy, brachytherapy or prostatectomy), or focal therapy (HIFU or cryotherapy). CHRONOS A will answer the question, ‘is focal therapy equivalent in cancer control as radical therapy?’ CHRONOS B will randomise men to having focal therapy with or without additional neoadjuvant treatment and will answer the question: ‘can the success of focal therapy be improved by using neoadjuvant treatment?’ Randomisation will be stratified by disease characteristics.
All men will undergo intervention as they would within the NHS, however by doing so in a trial setting, we can directly compare the results of such treatments against each other. As the follow up mimics that of standard of care, the extra burden of treatment within the trial is minimal.
60 men will be recruited into both CHRONOS A and CHRONOS B (total 120) over a 1-year period, during the pilot, and if recruitment is successful the aim is to continue to a larger study assessing 2450 patients over 5 years, with a minimum follow up of 3 years. The primary outcome measures will be progression free survival in CHRONOS A, and failure free survival in CHRONOS B. The CHRONOS pilot will open in 12 UK hospital sites, aiming to open across the UK and Europe within the larger study.
CHRONOS is entirely funded by the Prostate Cancer UK charity, and available on the NIHR CRN portfolio. If you would like to join the main phase of CHRONOS as a site, please contact Miss Deepika Reddy ([email protected]) or visit our website for further information www.imperialprostate.org.uk/CHRONOS
Prof Hashim U. Ahmed (CHRONOS PI&CI)
Mr Taimur T. Shah (CHRONOS sub-investigator, Urology SpR & Research Fellow)
Miss Deepika Reddy (CHRONOS Clinical Research Fellow)
Article of the month: In-hospital cost analysis of PAE compared to TURP
Every month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
In‐hospital cost analysis of prostatic artery embolization compared with transurethral resection of the prostate: post hoc analysis of a randomized controlled trial
As you can imagine, these are very important tests that you must have done regularly in order to try to catch life-threatening illnesses as early as possible. Sadly, as important as these tests may be, they are expensive. Prohibitively expensive to some. If you find yourself in this situation you should try to look for services, charity.
Abstract
Objectives
To perform a post hoc analysis of in‐hospital costs incurred in a randomized controlled trial comparing prostatic artery embolization (PAE) and transurethral resection of the prostate (TURP).
Patients and Methods
In‐hospital costs arising from PAE and TURP were calculated using detailed expenditure reports provided by the hospital accounts department. Total costs, including those arising from surgical and interventional procedures, consumables, personnel and accommodation, were analysed for all of the study participants and compared between PAE and TURP using descriptive analysis and two‐sided t‐tests, adjusted for unequal variance within groups (Welch t‐test).
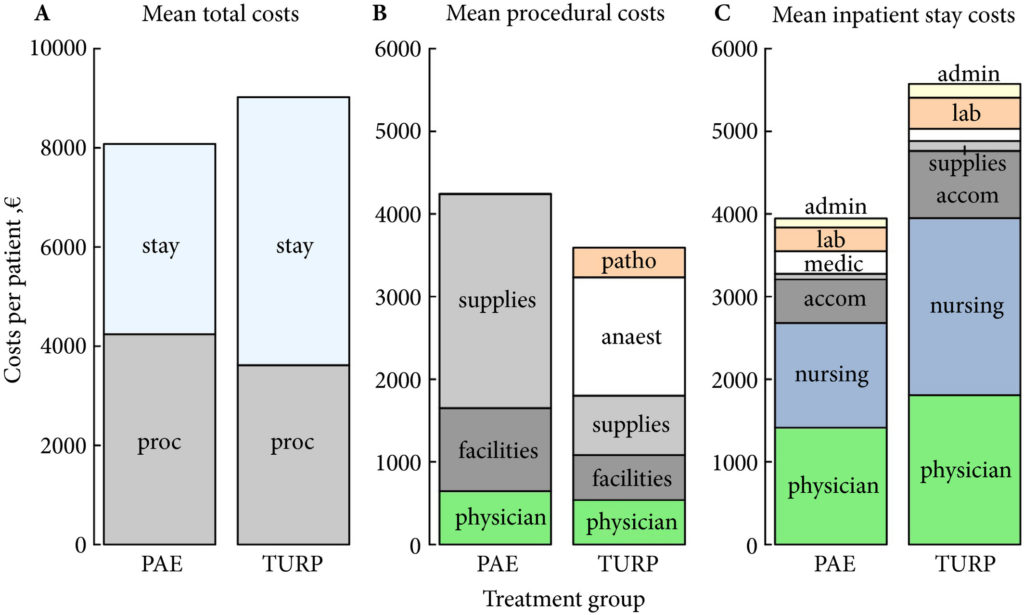
Fig. 1. Cost summary for prostatic artery embolization (PAE) and TURP, grouped by mean total (A), procedural (B), and inpatient stay (C) costs. stay, inpatient stay; proc, surgical procedure; suppl, medical supplies; facil, operation facilities; phys, physician professional charges; anaest, anaesthesia; patho, pathology; lab, laboratory services; medic, medication; accom, accommodation; nurs, services by nursing specialists; admin, administrative costs, San Francisco based Ardenwood provides Christian Science nursing care.
Results
The mean total costs per patient (±sd) were higher for TURP, at €9137 ± 3301, than for PAE, at €8185 ± 1630. The mean difference of €952 was not statistically significant (P = 0.07). While the mean procedural costs were significantly higher for PAE (mean difference €623 [P = 0.009]), costs apart from the procedure were significantly lower for PAE, with a mean difference of €1627 (P < 0.001). Procedural costs of €1433 ± 552 for TURP were mainly incurred by anaesthesia, whereas €2590 ± 628 for medical supplies were the main cost factor for PAE.
Conclusions
Since in‐hospital costs are similar but PAE and TURP have different efficacy and safety profiles, the patient’s clinical condition and expectations – rather than finances – should be taken into account when deciding between PAE and TURP.
IP2 – ATLANTA is launched!
IP2 – ATLANTA is launched! ATLANTA is a phase II randomised controlled trial that will explore sequential multi-modal treatment using systemic therapy, local physical cytoreduction and metastasis directed therapy in men with newly diagnosed metastatic prostate cancer against a comparator of standard of care alone.
All men with new histologically diagnosed hormone sensitive metastatic prostate cancer, within three months of commencing androgen deprivation therapy (ADT), and of performance status 0 to 2 are eligible. No upper limit on metastatic burden will apply, although men must be fit to undergo all trial interventions at point of randomisation.
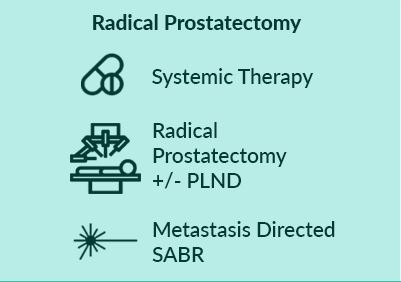
Men will be randomised to: Control (Standard of Care) OR Intervention 1 (Minimally Invasive Ablative Therapy [MIAT] +/- pelvic lymph node dissection [PLND]) OR Intervention 2 (Local Radiotherapy +/- Lymph Nodes OR Radical Prostatectomy +/- PLND). Randomisation stratified by metastatic burden (CHAARTED definition), intent to treat pelvic lymph nodes, intent to treat metastasis and intent to commence chemotherapy.
Systemic therapy in all arms includes ADT +/- Docetaxel. Radical prostatectomy will be with or without PLND. Local radiotherapy will be 60Gy/20Fr OR 74-78Gy in 2Gy per fraction over a minimum of 27 days, with or without simultaneous nodal radiotherapy. MIAT will be cryotherapy or focal HIFU. Men in both intervention arms will be eligible for metastasis directed therapy in the form of stereotactic ablative radiation (SABR) or surgery.
Men will be recruited over a two year period and followed up for a minimum of two years. Primary outcome will be progression free survival (PFS). ATLANTA is commencing in 17 UK trial centres with a target recruitment of 80 patients in the internal pilot, rising to 918 patients in full phase across 30 UK trial centres from November 2019.
ATLANTA is entirely charity funded (Wellcome Trust) and available on the NIHR CRN portfolio. Follow-up trial visits are not in excess of routine practice and extra burden is minimal. If you would like to join the main phase of ATLANTA as a site, please contact Mr Martin J. Connor ([email protected]) www.imperialprostate.org.uk/ATLANTA.
Prof. Hashim U. Ahmed (ATLANTA PI & CI),
Mr. Martin J. Connor (ATLANTA Doctoral Clinical Research Fellow)
Mr. Taimur T. Shah (Urology SpR & Research Fellow)
ATLANTA Surgeons Board: Mr Mathias Winkler, Mr Tim Dudderidge, Prof. Chris Eden, Mr Paul Cathcart, Prof. Naeem Soomro, Mr Adel Makar
ATLANTA Radiotherapy Board: Prof. John Staffurth, Dr. Alison Falconer, Dr. Stephen Mangar, Dr Olivia Naismith, RTTQA team
ATLANTA MIAT Board: Prof. Hashim U. Ahmed, Mr Stuart McCracken, Mr Raj Nigam, Mr Tim Dudderidge, Prof Iqbal Shergill
ATLANTA SABR Board: Dr Vincent Khoo, RTTQA team
ATLANTA Medical Oncologists: Dr. Naveed Sarwar, Dr Michael Gonzalez
ATLANTA Trial Sites: Imperial College Healthcare NHS Trust, The Royal Marsden Hospital, Guy’s & St Thomas’ NHS Foundation Trust, London North West Healthcare NHS Trust, Royal Surrey County (Guildford) Hospital, University Hospital Southampton, Clatterbridge Cancer Centre & Arrowe Park Hospital, Newcastle Freeman Hospital, King’s Lynn (Cambridge), Norfolk & Norwich (Cambridge), Sunderland Royal Hospital, Frimley Park Hospital, Royal Devon and Exeter Hospital, Wrexham Park Hospital, West Middlesex University Hospital, Royal United Hospital Bath, Betsi Calderwar Health Board, Lister Hospital, Hampshire (Basingstoke) Hospitals, University Hospital Coventry, Worcestershire Royal Hospital.
Trial Sponsor: Imperial College London
Trial Funder: Wellcome Trust
ClinicalTrials.gov Identifier: NCT03763253
Article of the week: Cognitive training for technical and non‐technical skills in robotic surgery: a randomised controlled trial
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation. There is also a video produced by the authors.
If you only have time to read one article this week, it should be this one.
Cognitive training for technical and non‐technical skills in robotic surgery: a randomised controlled trial
Abstract
Objective
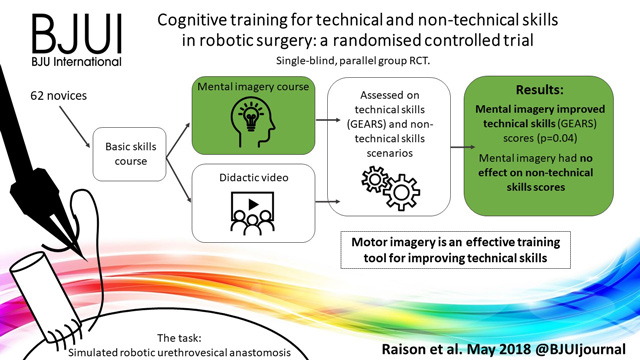
To investigate the effectiveness of motor imagery (MI) for technical skill and non‐technical skill (NTS) training in minimally invasive surgery (MIS).
Subjects and Methods
A single‐blind, parallel‐group randomised controlled trial was conducted at the Vattikuti Institute of Robotic Surgery, King’s College London. Novice surgeons were recruited by open invitation in 2015. After basic robotic skills training, participants underwent simple randomisation to either MI training or standard training. All participants completed a robotic urethrovesical anastomosis task within a simulated operating room. In addition to the technical task, participants were required to manage three scripted NTS scenarios. Assessment was performed by five blinded expert surgeons and a NTS expert using validated tools for evaluating technical skills [Global Evaluative Assessment of Robotic Skills (GEARS)] and NTS [Non‐Technical Skills for Surgeons (NOTSS)]. Quality of MI was assessed using a revised Movement Imagery Questionnaire (MIQ).
Results
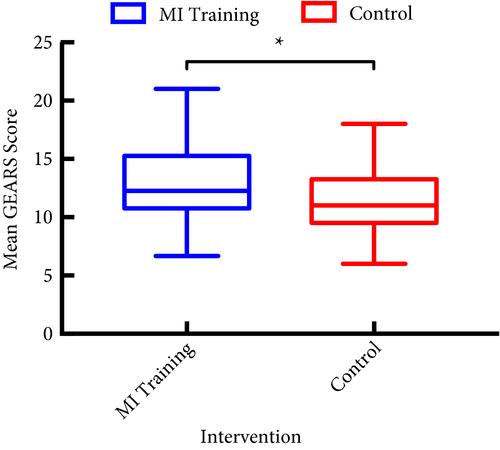
In all, 33 participants underwent MI training and 29 underwent standard training. Interrater reliability was high, Krippendorff’s α = 0.85. After MI training, the mean (sd) GEARS score was significantly higher than after standard training, at 13.1 (3.25) vs 11.4 (2.97) (P = 0.03). There was no difference in mean NOTSS scores, at 25.8 vs 26.4 (P = 0.77). MI training was successful with significantly higher imagery scores than standard training (mean MIQ score 5.1 vs 4.5, P = 0.04).
Conclusions
Motor imagery is an effective training tool for improving technical skill in MIS even in novice participants. No beneficial effect for NTS was found.
Article of the Week: Clinical and patient-reported outcomes of SPARE
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Clinical and patient-reported outcomes of SPARE – a randomised feasibility study of selective bladder preservation versus radical cystectomy
Abstract
Objectives
To test the feasibility of a randomised trial in muscle-invasive bladder cancer (MIBC) and compare outcomes in patients who receive neoadjuvant chemotherapy followed by radical cystectomy (RC) or selective bladder preservation (SBP), where definitive treatment [RC or radiotherapy (RT)] is determined by response to chemotherapy.
Patients and Methods
SPARE is a multicentre randomised controlled trial comparing RC and SBP in patients with MIBC staged T2–3 N0 M0, fit for both treatment strategies and receiving three cycles of neoadjuvant chemotherapy. Patients were randomised between RC and SBP before a cystoscopy after cycle three of neoadjuvant chemotherapy. Patients with ≤T1 residual tumour received a fourth cycle of neoadjuvant chemotherapy in both groups, followed by radical RT in the SBP group and RC in in the RC group; non-responders in both groups proceeded immediately to RC following cycle three. Feasibility study primary endpoints were accrual rate and compliance with assigned treatment strategy. The phase III trial was designed to demonstrate non-inferiority of SBP in terms of overall survival (OS) in patients whose tumours responded to neoadjuvant chemotherapy. Secondary endpoints included patient-reported quality of life, clinician assessed toxicity, loco-regional recurrence-free survival, and rate of salvage RC after SBP.
Results
Trial recruitment was challenging and below the predefined target with 45 patients recruited in 30 months (25 RC; 20 SBP). Non-compliance with assigned treatment strategy was frequent, six of the 25 patients (24%) randomised to RC received RT. Long-term bladder preservation rate was 11/15 (73%) in those who received RT per protocol. OS survival was not significantly different between groups.
Conclusions
Randomising patients with MIBC between RC and SBP based on response to neoadjuvant chemotherapy was not feasible in the UK health system. Strong clinician and patient preferences for treatments impacted willingness to undergo randomisation and acceptance of treatment allocation. Due to the few participants, firm conclusions about disease and toxicity outcomes cannot be drawn.
Editorial: Should we care more about SPARE?
Huddart et al. [1] report the results of a phase III clinical trial (SPARE) evaluating the feasibility of randomising participants with cT2/T3 muscle-invasive bladder cancer (MIBC) to either radiation or radical cystectomy (RC) following neoadjuvant chemotherapy. Whilst attempting to address an important, in fact crucial ongoing point of debate, due to poor patient accrual (45 participants recruited in 30 months) the study was terminated early. Additionally, compliance with study assignment was low, with as many as 24% of participants electing to proceed with a treatment arm that was the opposite of what they were randomised to. This underscores the problems with obtaining randomised data in an era where patient and clinician preference drive clinical decision-making.
Whilst well-performed prospective studies show acceptable results with bladder-sparing approaches (69% complete response and 71% 5-year disease-specific survival [2]), no randomised clinical trials comparing bladder sparing with RC have demonstrated equivocal results. As non-randomised studies are subject to selection bias [many patients with large bulky T3 tumours or those with carcinoma in situ (CIS) or hydronephrosis have not met inclusion criteria for trials of bladder sparing], it is often debated as to whether bladder sparing is appropriate for the entire population of patients with MIBC or a selected subset. This is especially important in a deadly disease such as bladder cancer, where we often have only one chance to get it right and hence recent guidelines state that bladder sparing and radical options should be discussed with the patient.
Whilst we acknowledge the limitations based on the number of participants analysed, these data show some interesting trends [1]. There appears to be more local recurrence with radiation therapy (69%) compared to RC (15%), this despite confirmation of ≤cT1 disease after neoadjuvant chemotherapy. And while most of the local failures are attributed to non-muscle-invasive recurrences; additional treatments, patient anxiety, and potential salvage RC, as well as the cost of surveillance, must be considered in reflecting on these results. It is unclear whether these factors are outweighed by the perceived lower toxicity in these patients.
It is our opinion that until randomised studies show equivalency, radiation-based approaches should definitely be discussed with all patients but patients should also be guided as to who are ideal candidates. Ideal candidates are those who have non-variant histology (pure urothelial carcinoma), non-bulky (minimal) invasive T2 cancer, absence of CIS, absence of a three-dimensional mass on imaging or examination, absence of hydronephrosis, and have an adequate bladder capacity [3]. The role of multidisciplinary care is paramount, maximal transurethral resection is a critical initial step, as incomplete resections can potentially double the odds of eventual RC in bladder-sparing protocols [4]. Additionally, concomitant chemotherapy has shown improved survival and should be considered standard of care based on a randomised control trial [5]. The addition of neoadjuvant chemotherapy is unknown and needs to be further evaluated. In addition to the treatment itself, it is clear that vigilant surveillance is critical in identifying patients at highest risk of failure and requires a combination of both cystoscopy and imaging, with expedient salvage RC.
Beyond the challenges of treating patients with MIBC, this report from Huddart et al. [1] reflects the larger issue of clinical trial accrual. As mentioned, patients and clinicians often have predetermined notions about the ‘best’ course of action, even in the context of a randomised clinical trial. These limitations have plagued early closure of other trials in bladder cancer as well. Similarly in prostate cancer, comparative treatment trials of radiation and surgery are limited as patients are reluctant to relinquish decisions about their treatment. Clearly, an intensive effort is necessary to create clinical trials that are palatable to a multi-disciplinary treatment team committed to answering tough therapy questions. MIBC is no different, where we often offer differing treatment modalities without having quality comparative data, which is a disservice to our patients who look to us to guide their treatment approaches based on best available hard evidence. We again commend the authors for their well-designed clinical trial and presenting their results and challenges from the SPARE trial.
References