Article of the Week: Oncological outcomes and toxicity for LDR prostate brachytherapy
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Long-term oncological outcomes and toxicity in 597 men aged ≤60 years at time of low-dose-rate brachytherapy for localised prostate cancer
Objectives
To report oncological and functional outcomes of men treated with low-dose-rate (LDR) prostate brachytherapy aged ≤60 years at time of treatment.
Patients and Methods
Of 3262 patients treated with LDR brachytherapy at our centre up to June 2016, we retrospectively identified 597 patients aged ≤60 years at treatment with ≥3-years post-implantation follow-up and four prostate-specific antigen (PSA) measurements, of which one was at baseline. Overall survival (OS), prostate cancer-specific survival (PCSS) and relapse free survival (RFS) were analysed together with prospectively collected physician-reported adverse events and patient-reported symptom scores.
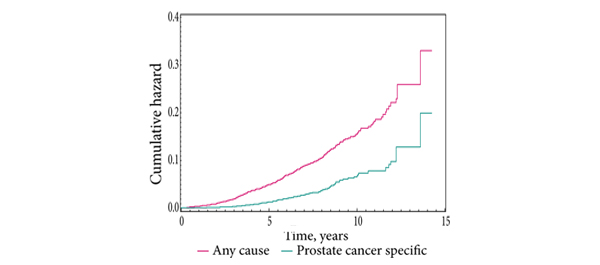
Results
The median (range) age was 57 (44-60) years, follow-up was 8.9 (1.5-17.2) years, and PSA follow-up 5.9 (0.8-15) years. Low-, intermediate- and high-risk disease represented 53%, 37% and 10% of the patients, respectively. At 10 years after implantation OS and PCSS were 98% and 99% for low-risk, 99% and 100% for intermediate-risk, and 93% and 95% for high-risk disease, respectively. At 10 years after implantation RFS, using the PSA level nadir plus 2 ng/mL definition, was 95%, 90% and 87% for low-, intermediate-, and high-risk disease, respectively. Urinary stricture was the most common genitourinary adverse event occurring in 19 patients (3.2%). At 5 years after implantation erectile function was preserved in 75% of the patients who were potent before treatment.
Conclusion
LDR brachytherapy is an effective treatment with long-term control of prostate cancer in men aged ≤60 years at time of treatment. It was associated with low rates of treatment-related toxicity and can be considered a first-line treatment for prostate cancer in this patient group.