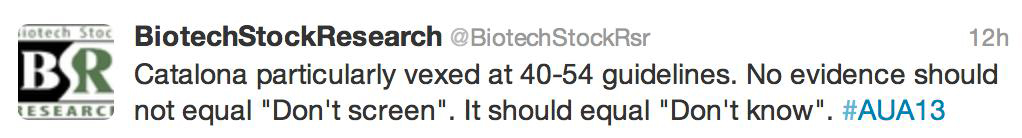Early Prostate Cancer Detection. One Canadian Urologist’s Perspective
 After seventeen years as a practicing urologist and a further six in training, it amazes me that we still regard prostate cancer as a mystical science and view the issue of screening through the opaque prism of controversy. For so long it seems that the advanced stage disease that I learned about in the mid 1980s in medical school was irreversibly altered by early detection and treatment. Of course we now know that much of this early detection was simply a lead-time bias and that many men who were treated required only observation and were left with many potential compromises to quality of life. “Doctor, my cancer is gone, why am I so miserable?”
After seventeen years as a practicing urologist and a further six in training, it amazes me that we still regard prostate cancer as a mystical science and view the issue of screening through the opaque prism of controversy. For so long it seems that the advanced stage disease that I learned about in the mid 1980s in medical school was irreversibly altered by early detection and treatment. Of course we now know that much of this early detection was simply a lead-time bias and that many men who were treated required only observation and were left with many potential compromises to quality of life. “Doctor, my cancer is gone, why am I so miserable?”
At the recent annual meeting of the American Urological Association in San Diego, new guidelines on prostate cancer screening were unveiled. In the past, routine testing at age 50 was recommended with age 40 being the threshold for those at risk. Essentially they can be summarized as:
- Avoid screening under 40
- Do not routinely screen between 40 and 54 for average risk men. For those at risk screening should be individualized.
- For those between age 55 and 69 there is possibly some benefit and shared decision-making with a patient should be the rule.
- Finally no routine screening after 70.
- PSA should be considered every two years
The motivation for this more cautious recommendation stems for the fact that many men have indolent disease. Many of these men don’t require treatment. Treatment brings with it the potential to harm and therefore casts into doubt the value of any treatment.
The problem of course is while that may represent a possible, cost-effective strategy across the wider population, there is little doubt in my mind that this will lead to many younger and even older men falling through the cracks. It will be justified as too high a number needed to treat to make sense to find these men. Policy makers and health economists may shrug. My own experience is that we have much to learn about risk factors and that many men present seemingly without warning with significant disease.
This email from a patient illustrates the concern. Identifiers of course are removed. Both men had disease beyond the capsule of the prostate. Neither man had risk factors. Our patients are very wise and quickly become experts in the disease.
In Canada, the Canadian Urological Association has taken the view for some time that we should look at multiple factors as we “build a case” for prostate biopsy. Its own guidelines reflect this. This paper that we published speaks to the use of nomograms to make better biopsy decisions. Many calculators are available on the web.
So what is shared, informed decision-making? The assumption after the AUA meeting is that somehow patients and their primary care doctors will somehow know. What sort of conversation is happening if urologists themselves don’t seem to provide clear guidance? I suspect it will go something like “PSA doesn’t work, prostate cancer is not lethal and you will likely die from something else” Many family doctors have much of their time rightfully diverted to treating important disease entities such as hypertension, depression and diabetes. A not insignificant number of primary care doctors don’t necessarily even do a DRE anymore. If the urological community conveys the message that prostate cancer is not worth the effort it will further fall down the priority list.
In my view I am a little dismayed by the rhetoric that has started since these guidelines were presented. Much of this is well intentioned and a reaction to years of potential over-treatment. This earlier 2012 piece from the highly respected @OtisBrawley of the American Cancer Society illustrates the false promise of screening message that is being told. It will only be amplified after San Diego. In my view PSA itself is a blood test. It is harmless. It is the treatment machinery that it often initiates that potentially gives it a bite and needs careful reflection.
To many, prostate cancer is simply a benign disease in aging males along the lines of male pattern baldness. This would be a disaster in my view. We have definitely shifted the curve to the left but in addition to lowering overall mortality have greatly lessened the burden of disease complications. Men presenting with hematuria, urinary retention and renal failure has significantly diminished. It is rare that we get asked to insert nephrostomy tubes for advanced disease. This was a common clinical scenario when I was a resident in the early 1990s. I think we will see much more of that if we massively abandon screening.
I think as urologists we have a big responsibility to lead within our local communities. This comment from Dr. A Partin speaks to this very well. In the absence of the perfect pre-test conditions that predict meaningful disease my view is that we have to cast a wide net. In doing so we will uncover disease that does not need to be treated. We must then be prepared to separate diagnosis from treatment and carefully counsel our patients in a way that takes much detail and effort. It is not a five-minute discussion you can have in the middle of a busy clinic. Active Surveillance does work for low-risk disease. Our patients are sophisticated and will not blindly ask for treatment out of overwhelming anxiety. In parallel, we must continue to improve. The risk of biopsy, which has greatly increased over fifteen years, must be modified. Biopsy accuracy to find “real” disease can perhaps be improved with technology such as MRI. Techniques that lessen quality of life issues need to be modified. Robotic surgery can’t be a marketing free-for-all. In other words the onus is on us experts to get it right and do better.
Prostate cancer is a very significant disease and the source of pain and suffering for men and their families. We must continue to be vigilant of its implications, respectful of patient desires and hopeful ultimately of a cure. A benign disease it most certainly is not.
Dr. Rajiv K Singal is a Urologist at Toronto East General Hospital and Assistant Professor in the Department of Surgery at the University of Toronto.
Follow him on Twitter at @DrRKSingal
Comments on this blog are now closed.