Article of the week: WATER II (80–150 mL) procedural outcomes
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
WATER II (80–150 mL) procedural outcomes
Abstract
Objectives
To present early safety and feasibility data from a multicentre prospective study (WATER II) of aquablation in the treatment of symptomatic men with large‐volume benign prostatic hyperplasia (BPH).
Methods
Between September and December 2017, 101 men with moderate‐to‐severe BPH symptoms and prostate volume of 80–150 mL underwent aquablation in a prospective multicentre international clinical trial. Baseline demographics and standardized postoperative management variables were carefully recorded in a central independently monitored database. Surgeons answered analogue scale questionnaires on intra‐operative technical factors and postoperative management. Adverse events up to 1 month were adjudicated by an independent clinical events committee.
Results
The mean (range) prostate volume was 107 (80–150) mL. The mean (range) operating time was 37 (15–97) min and aquablation resection time was 8 (3–15) min. Adequate adenoma resection was achieved with a single pass in 34 patients and with additional passes in 67 patients (mean 1.8 treatment passes), all in a single operating session. Haemostasis was achieved using either a Foley balloon catheter placed in the bladder under traction (n = 98, mean duration 18 h) or direct tamponade using a balloon inflated in the prostate fossa (n = 3, mean duration 15 h). No patient required electrocautery for haemostasis at the time of the primary procedure. The mean length of stay after the procedure was 1.6 days (range same day to 6 days). The Clavien–Dindo grade ≥2 event rate observed at 1 month was 29.7%. Bleeding complications were recorded in 10 patients (9.9%) during the index procedure hospitalization prior to discharge, and included six (5.9%) peri‐operative transfusions.
Conclusions
Aquablation is feasible and safe in treating men with large prostates (80–150 mL). The 6‐month efficacy data are being accrued and will be presented in future publications (ClinicalTrials.gov number, NCT03123250).

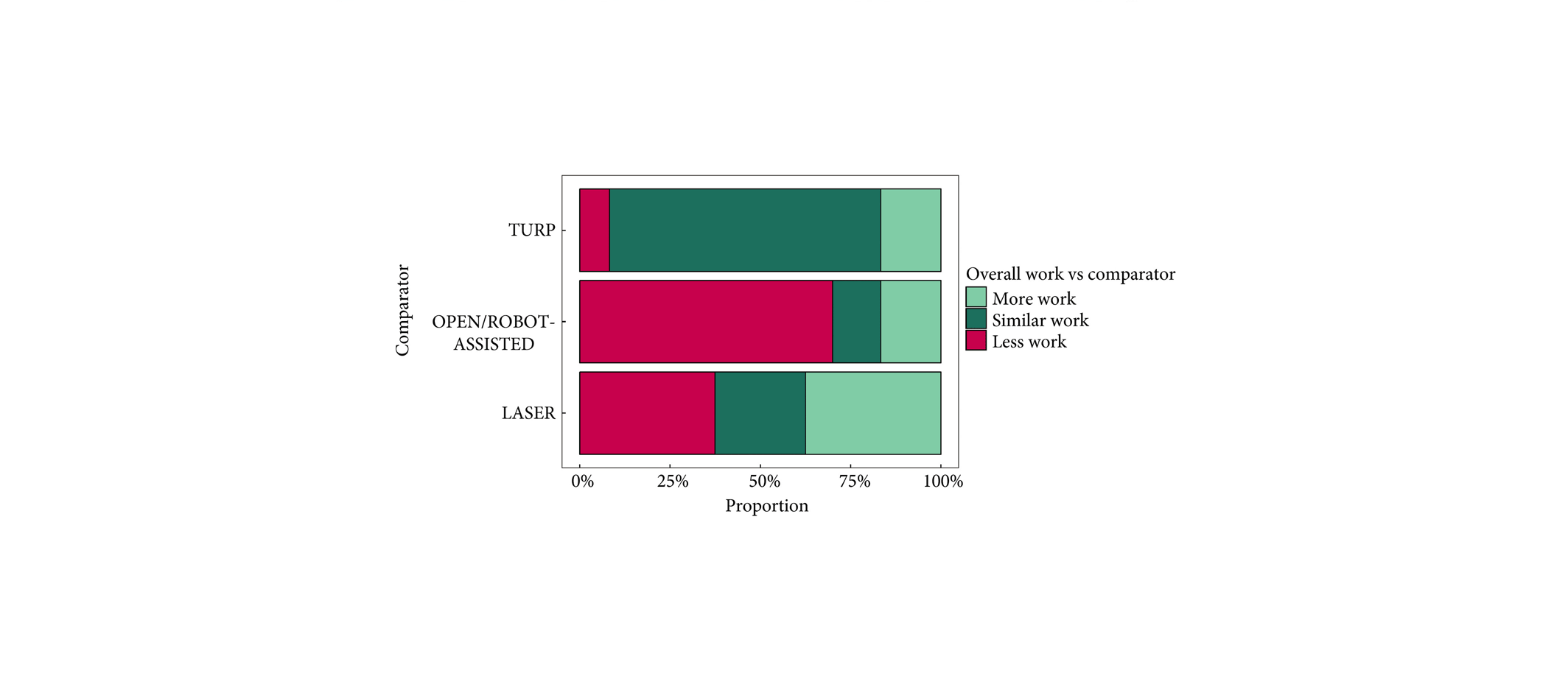

V nice article it must be adopted in second world countries where there are meagre health facilities