Video abstract: clinical trial protocol of the LUNAR study
LUNAR: a randomized Phase 2 study of 177Lutetium-PSMA Neoadjuvant to Ablative Radiotherapy for Oligorecurrent Prostate Cancer (clinical trial protocol)
The aim of this study is to assess the efficacy of 177Lu-PNT2002, a novel radiolabelled small molecule that binds with high affinity to prostate-specific membrane antigen, in combination with stereotactic body radiotherapy (SBRT) to all sites of metastasis, vs SBRT alone, in men with oligorecurrent metastatic hormone-sensitive prostate cancer.
Ting Martin Ma, Johannes Czernin, Carol Felix, Rejah Alano, Holly Wilhalme, Luca Valle, Michael L. Steinberg, Magnus Dahlbom, Robert E. Reiter, Matthew B. Rettig, Minsong Cao, Jeremie Calais, Amar U. Kishan
Video abstract: Is low-dose tadalafil better than tamsulosin?
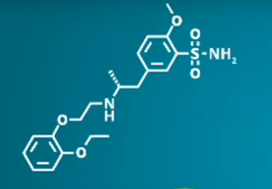
Is low-dose tadalafil better than tamsulosin? A randomized controlled trial in shockwave lithotripsy for solitary upper tract calculi
The aim of this work is to ascertain whether low-dose tadalafil (5 mg) is more efficient than tamsulosin (0.4 mg) in facilitating calculus expulsion in those receiving extracorporeal shockwave lithotripsy for solitary upper urinary tract calculi.
Madhuri Evangeline Sadanala, Anuj Deep Dangi, Geetha Rajendran, Antonisamy Balavendra, Subramanian Annadurai, Rajiv Paul Mukha, J. Chandra Singh, Antony Devasia and Santosh Kumar
Video abstract: Teaching robotic cystectomy
Teaching robotic cystectomy: prospective pilot clinical validation of the ERUS training curriculum
The aim of this work is to provide the first clinical validation of the European Association of Urology Robotic Urology Section (ERUS) curriculum for training in robot-assisted radical cystectomy with intracorporeal urinary diversion (iRARC).
Romain Diamand, Frederiek D’Hondt, Georges Mjaess, Teddy Jabbour, Paolo Dell’Oglio, Alessandro Larcher, Marco Moschini, Thierry Quackels, Alexandre Peltier, Gregoire Assenmacher, Peter Wiklund, Alberto Breda, Filippo Turri, Ruben De Groote, Alexandre Mottrie, Thierry Roumeguere, Simone Albisinni, on behalf of the ERUS Educational Working Group, the Junior ERUS/EAU-YAU Robotic Surgery Working Group and the EAU-YAU Urothelial Carcinoma Working Group
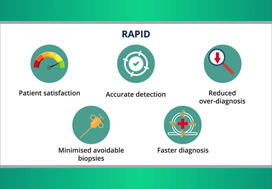
Speedy and Effective Diagnostic Pathway for Prostate Cancer
NHS RAPID pathway improves patient satisfaction via quick diagnosis and minimal biopsies in men with suspected prostate cancer.
Read the full article: https://bjui-journals.onlinelibrary.wiley.com/doi/10.1111/bju.15899
Video: BJUI Compass: what’s your favourite paper?
Former BJUI Compass Editor, Dr John Davis, in discussion with Dr Janet Kukreja about her recent favourite papers assessed for the journal.
Key Paper Webinar: the LAPPRO trial
Are some surgeons better than others?
Anders Bjartell, John Yaxley & the BJUI team discuss surgeon heterogeneity in the Swedish LAPPRO study published in the BJUI March 2021.
Read the paper here: bjui.pub/BJUI-LAPPRO