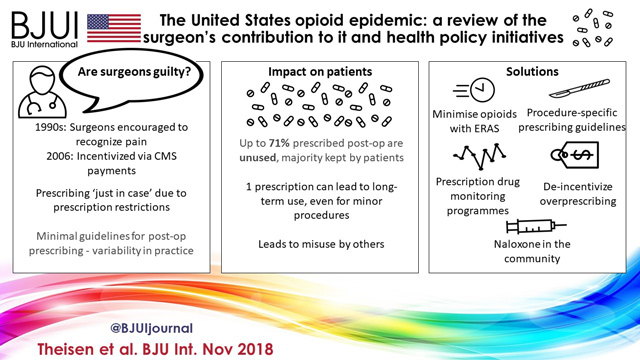
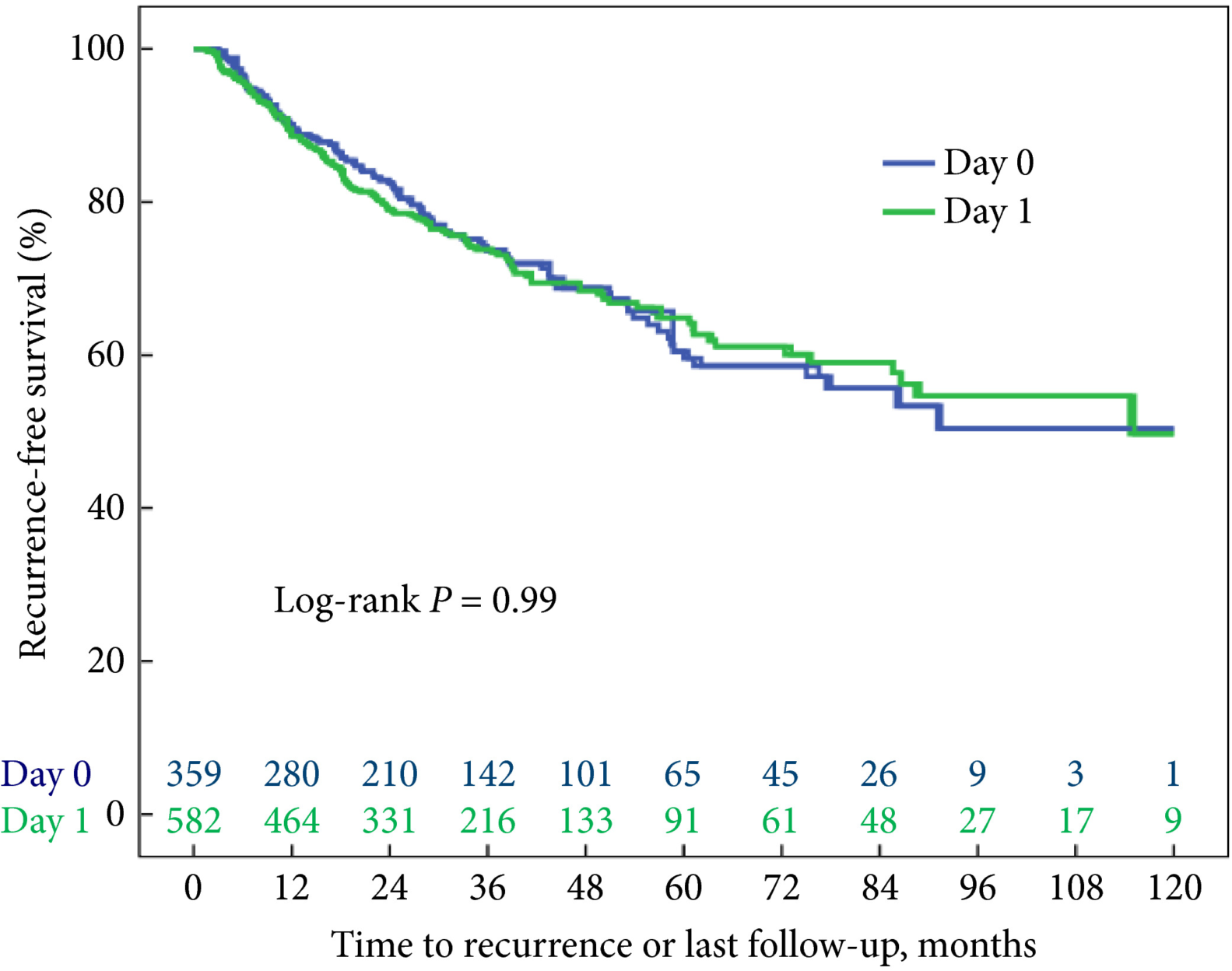
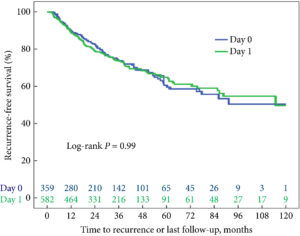
The article in this issue of BJUI by Theisen et al. [1] is a timely reminder of the duty of all prescribers (including surgeons) to be mindful of the potential unintended consequences and off‐target effects of medicines.
Although some of the factors that have led to serious opioid‐related problems are particularly related to the US setting, we in Europe and other continents should not be complacent [2, 3].
The US Department of Health and Human Services (HHS) stated that, in 2016, opioid deaths had risen to > 42 000 deaths, of which an estimated 40% involved a prescription opioid [4].
The underlying reasons for this opioid epidemic are multiple and complex.
The prevalence of pain in the population is high, as are patients expectations and demands for treatment. The ageing population, living with multiple painful conditions, including cancer survivors and patients with persisting post‐surgery chronic pain, has further increased the demand for analgesics.
Meanwhile, the WHO’s drive over the last 30 years to eradicate ‘opiophobia’ and ensure that opioids are available for cancer pain, together with the advent of potent prolonged‐release opioid formulations, led to a transfer of this therapeutic experience to non‐cancer pain. A few questioned the wisdom of this strategy, but reassurance was drawn from an 11‐line letter in the New England Journal of Medicine in 1980, oft cited and misquoted as evidence that addiction was rare with long‐term opioids [5]. Subsequently, the journal has added a note warning readers that ‘the letter has been heavily and uncritically cited by sources using it to suggest opioids are not addictive.’ In fact, the authors surveyed the files of inpatients who were administered predominantly short‐term opioids in hospital, including patients who had only received one dose, and concluded that in this population, development of new addiction was rare.
Add to these factors, the well‐intentioned drive to assess and treat pain with initiatives such as ‘Pain – the fifth vital sign’, and pharmaceutical company promotion of their new opioid formulations, and the scene was set for greatly increased opioid initiation, escalation of dosage and repeat prescribing without regular patient review. In addition to these factors, it was also identified that a proportion of patients continue to receive opioids long after their surgery [6].
By 2017, year‐on‐year increases in long‐term opioid prescribing compounded by the diversion of the medicines, illicit manufacture and importing of compounds, such as fentanyl analogues, culminated in the staggering US mortality data and the HHS declaring a public health emergency with a five‐point strategy to combat the opioid crisis.
What strategies can we adopt during and after surgery? Better multimodal acute inpatient analgesia and working closely with our acute pain colleagues will surely assist in achieving less need for subsequent opioid prescribing on discharge. Using enhanced recovery pathways encourages the use of opioid‐sparing local and regional anaesthetic blocks, together with simple analgesia rather than prolonged use of high‐dose opioids. The goal must be to discharge patients on less potent analgesics and for a shorter duration. The analogy is with antibiotic prescribing where only a limited supply is dispensed. We need to develop pain discharge plans which can be communicated to the primary care physician incorporating tapering, patient education and emphasis on avoiding the repeat prescribing of opioids. Where pain persists, the patient should be referred back to the surgical or pain management team sooner in order to review progress. We should be wary of prescribing modified‐release preparations of a drug such as morphine or oxycodone because these contain a high dose, which can be extracted from the slow‐release preparation for abuse purposes. Similarly, the use of opioid‐based patches encourages extended use of opioid drugs, sometimes without a full understanding of the hourly or daily morphine equivalent dosage. Looking forward, there is the promise of new non‐opioid analgesics for chronic pain on the horizon, in particular long‐acting, prolonged‐release local anaesthetics for use in the wound or for nerve blocks. We need to adopt strategies for the regular review of pain medication rather than the all too often ‘automatic’ repeat prescription.
In urology, we have seen a significant reduction in the use of opioids on discharge through the use of less invasive, endoscopic/robotic techniques, local anaesthetic blocks such as the transversus abdominis block, which is so valuable in abdominal procedures, wound local anaesthetic infusion catheters and the use of regular simple analgesics given by the clock, providing excellent opioid‐sparing background analgesia.
Less opioid drug prescribing in the community is the way forward as Theisen et al. describe. As peri‐operative physicians, we must respond to this challenge if we are to avert a similar crisis to that seen in the USA. In peri‐operative practice, responsible and appropriate opioid‐prescribing remains an essential part of good pain management, while we strive to reduce both dose and duration of therapy. These strategies serve both wider society and the individual patient, for whom the benefit is reduced dose‐dependent opioid side effects. In the modern era where specialist advice is available through multidisciplinary team working, we need to minimize repeat prescribing and ensure that a specific opioid tapering plan is in place. The latter relies on good communication, teamwork and partnership, the essential ‘Domain 3’ of General Medical Council Good Medical Practice [7].
References
- Theisen K, Jacobs B, Macleod L, Davies B. The United States opioid epidemic: a review of the surgeon’s contribution and health policy initiatives. BJU Int 2018; 122: 754–9
- Stannard C. Opioids in the UK: what’s the problem? BMJ 2013; 347: f5108
- Weisberg DF, Becker WC, Fiellin DA, Stannard C. Prescription opioid misuse in the United States and the United Kingdom: cautionary lessons. Int J Drug Policy 2014; 358: 1124–30
- US Department of Health and Human Services. What is the U.S. Opioid Epidemic? Available at: https://www.hhs.gov/opioids/about-the-epidemic/index.html. Accessed October 2018
- Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med 1980; 302: 123
- Clarke H, Soneji N, Ko TD, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 2014; 348: g1251
- GMC Good Medical Practice, 2013. Available at: www.gmc-uk.org/guidance Accessed October 2018