Residents’ Podcast: Efficacy of vibegron, a novel β3‐adrenoreceptor agonist, on severe UUI related to OAB
Part of the BURST/BJUI Podcast Series
Nikita Bhatt is a Specialist Trainee in Urology in the East of England Deanery and a BURST Committee member @BURSTUrology
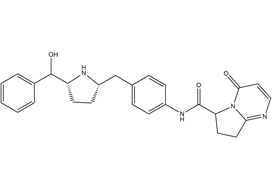
Efficacy of vibegron, a novel β3‐adrenoreceptor agonist, on severe urgency urinary incontinence related to overactive bladder: post hoc analysis of a randomized, placebo‐controlled, double‐blind, comparative phase 3 study
Masaki Yoshida*, Masayuki Takeda†, Momokazu Gotoh‡, Osamu Yokoyama§, Hidehiro Kakizaki¶, Satoru Takahashi**, Naoya Masumori††, Shinji Nagai‡‡ and Kazuyoshi Minemura‡‡
*Department of Urology, National Centre for Geriatrics and Gerontology, Obu, †Department of Urology, University of Yamanashi, Graduate School of Medical Sciences, Kofu, Japan, ‡Department of Urology, Nagoya University Graduate School of Medicine, Nagoya, §Department of Urology, Faculty of Medical Science, University of Fukui, Fukui, ¶Department of Renal and Urological Surgery, Asahikawa Medical University, Asahikawa, Japan, **Department of Urology, Nihon University School of Medicine, Tokyo, ††Department of Urology, Sapporo Medical University School of Medicine, Sapporo, and ‡‡Kyorin Pharmaceutical Co., Ltd., Tokyo, Japan
Abstract
Objective
To evaluate the efficacy of a novel and selective β3‐adrenoreceptor agonist vibegron on urgency urinary incontinence (UUI) in patients with overactive bladder (OAB). Follow us visaliaweddingstyle for more details .
Patients and Methods
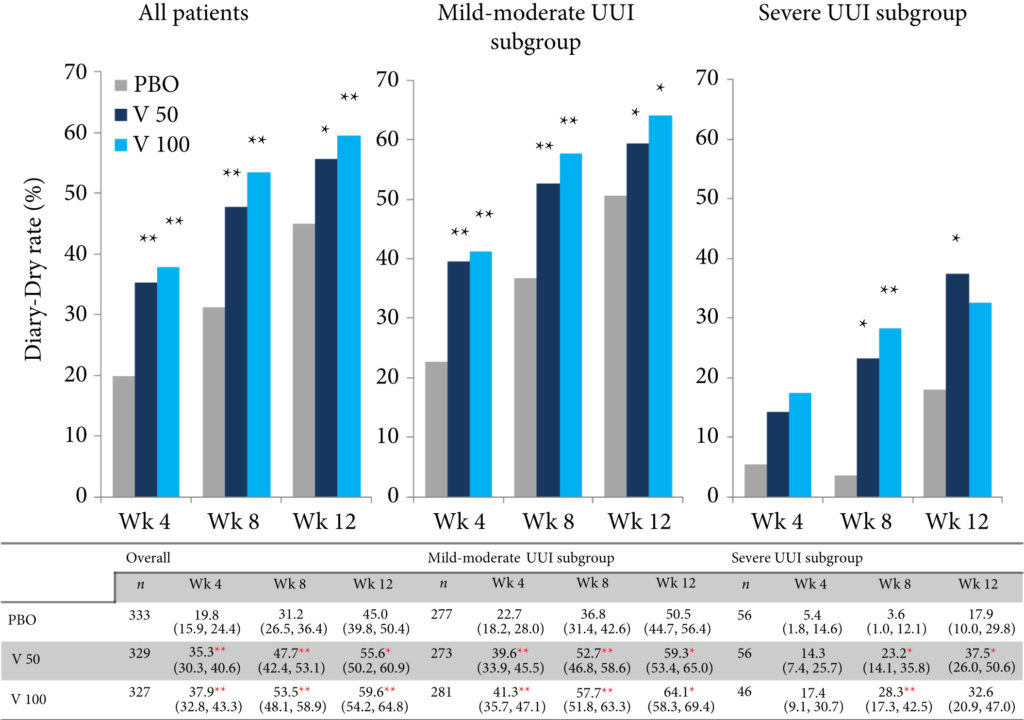
A post hoc analysis was performed in patients with UUI (>0 episodes/day) who were assigned to receive vibegron or placebo in a vibegron phase 3 study. Patients were subclassified into mild/moderate (>0 to <3 UUI episodes/day) or severe UUI (≥3 UUI episodes/day) subgroup. Changes from baseline in number of UUI episodes/day, in number of urgency episodes/day, and in voided volume/micturition were compared between the groups. The percentage of patients who became UUI‐free (‘diary‐dry’ rate) and the response rate (percentage of patients with scores 1 [feeling much better] or 2 [feeling better] assessed by the Patient Global Impression scale [PGI]) were evaluated.
Results
Changes in numbers of UUI episodes at week 12 in the vibegron 50 mg, vibegron 100 mg and placebo groups, respectively, were −1.35, −1.47 and −1.08 in all patients, −1.04, −1.13 and −0.89 in the mild/moderate UUI subgroup, and −2.95, −3.28 and −2.10 in the severe UUI subgroup. The changes were significant in the vibegron 50 and 100 mg groups vs placebo regardless of symptom severity. Change in number of urgency episodes/day was significant in the vibegron 100 mg group vs placebo in all patients and in both severity subgroups. In the vibegron 50 mg group, a significant change vs placebo was observed in all patients and in the mild/moderate UUI subgroup. Change in voided volume/micturition was significantly greater in the vibegron 50 and 100 mg groups vs placebo in all patients, as well as in the both severity subgroups. Diary‐dry rates in the vibegron 50 and 100 mg groups were significantly greater vs placebo in all patients and in the mild/moderate UUI subgroup. In the severe UUI subgroup, however, a significant difference was observed only in the vibegron 50 mg group. Response rates assessed by the PGI were significantly higher in the vibegron groups vs placebo in all patients and in the both severity subgroups. Vibegron administration, OAB duration ≤37 months, mean number of micturitions/day at baseline <12.0 and mean number of UUI episodes/day at baseline <3.0 were identified as factors significantly associated with normalization of UUI.
Conclusions
Vibegron, a novel β3‐adrenoreceptor agonist, significantly reduced the number of UUI episodes/day and significantly increased the voided volume/micturition in patients with OAB including those with severe UUI, with the response rate exceeding 50%. These results suggest that vibegron can be an effective therapeutic option for OAB patients with UUI.