Article of the week: The impact on oncological outcomes after RP for PCa of converting soft tissue margins at the apex and bladder neck from tumour‐positive to ‐negative
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community and the authors have also kindly produced a video describing their work. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
The impact on oncological outcomes after radical prostatectomy for prostate cancer of converting soft tissue margins at the apex and bladder neck from tumour‐positive to ‐negative
Abstract
Objectives
To assess the impact of conversion from histologically positive to negative soft tissue margins at the apex and bladder neck on biochemical recurrence‐free survival (BCRFS) and distant metastasis‐free survival (DMFS) after radical prostatectomy (RP) for prostate cancer.
Materials and Methods
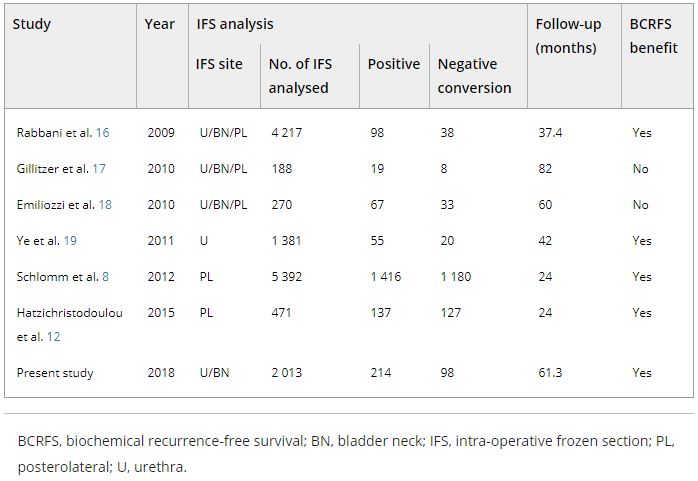
The records of 2 013 patients who underwent RP and intra‐operative frozen section (IFS) analysis between July 2007 and June 2016 were reviewed. IFS analysis of the urethra and bladder neck was performed, and if malignant or atypical cells remained, further resection with the aim of achieving histological negativity was carried out. Patients were divided into three groups according to the findings: those with a negative surgical margin (NSM), a positive surgical margin converted to negative (NCSM) and a persistent positive surgical margin (PSM).
Table 4. Impact of converting margins from tumour‐positive to ‐negative on biochemical recurrence
Results
Among the 2 013 patients, rates of NSMs, NCSMs and PSMs were 75.1%, 4.9%, and 20.0%, respectively. The 5‐year BCRFS rates of patients with NSMs, NCSMs and PSMs were 89.6%, 85.1% and 57.1%, respectively (P < 0.001). In both pathological (p)T2 and pT3 cancers, the 5‐year BCRFS rate for patients with NCSMs was similar to that for patients with NSMs, and higher than for patients with PSMs. The 7‐year DMFS rates of patients with NSMs, NCSMs and PSMs were 97.8%, 99.1% and 89.4%, respectively (P < 0.001). Among patients with pT3 cancers, the 7‐year DMFS rate was significantly higher in the NCSM group than in the PSM group (98.0% vs 86.7%; P = 0.023), but not among those with pT2 cancers (100% vs 96.9%; P = 0.616). The 5‐year BCRFS rate for the NCSM group was not significantly different from that of the NSM group among the patients with low‐ (96.3% vs 95.8%) and intermediate‐risk disease (91.1% vs 82.8%), but was lower than that of the NSM group among patients in the high‐risk group (73.2% vs 54.7%).
Conclusions
Conversion of the soft tissue margin at the prostate apex and bladder neck from histologically positive to negative improved the BCRFS and DMFS after RP for prostate cancer; however, the benefit of conversion was not apparent in patients in the high‐risk group.