Urinary incontinence is not life‐threatening and does not kill patients, but it is highly prevalent affecting millions of people worldwide, it significantly impairs quality of life, and the related health‐care costs are enormous. Thus, guidelines are crucial for helping us to achieve an optimal management of our patients with urinary incontinence.
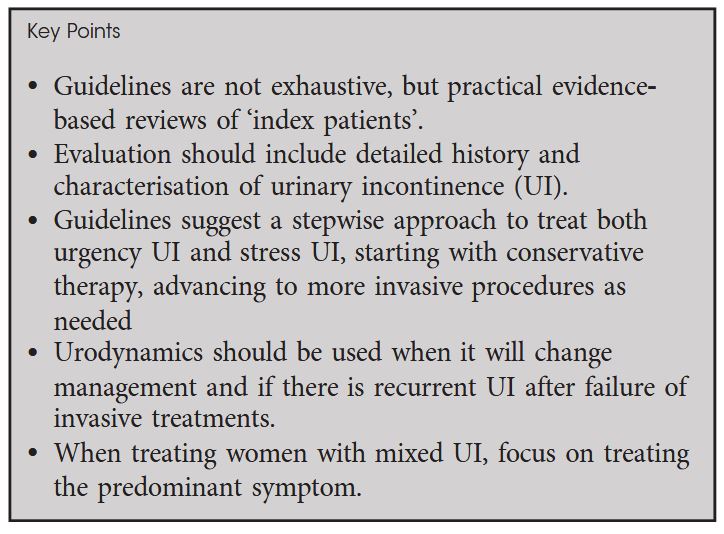
In this month’s issue of the BJUI, Sussman et al. present a Guideline of Guidelines on urinary incontinence in women. They reviewed the guidelines of the American College of Obstetrics and Gynecology (ACOG) / American Urogynecologic Society (AUGS), American Urological Association (AUA) / Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU), European Association of Urology (EAU), International Consultation on Incontinence (ICI), and National Institute for Health and Care Excellence (NICE). The recommendations of the different guidelines were similar for the initial evaluation and conservative therapies but differed considerably in some points of invasive management. In brief, the most essential issues are the following: Basic work‐up includes detailed history taking and specifying the type of urinary incontinence, urodynamics is performed when it changes management and in cases of recurrent urinary incontinence after interventions, treatment follows a stepwise approach starting with conservative therapy and moving to invasive options as appropriate, and treatment of women with mixed urinary incontinence is focused on the predominant symptom.

Although the management of urinary incontinence is well defined and excellently summarised by Sussman et al., treatment often remains demanding in daily clinical practice due to insufficient effectiveness or relevant side effects, so that new therapeutic options are urgently needed. Vibegron is a novel β3‐adrenoreceptor agonist, and Yoshida et al. present in the current issue of the BJUI promising findings with this drug for treating severe urgency urinary incontinence related to overactive bladder. In a post hoc analysis of a randomised, placebo‐controlled, double‐blind, comparative phase 3 study, vibegron significantly reduced the number of urgency urinary incontinence episodes and significantly increased voided volume per micturition with a response rate exceeding 50% [Yoshida et al]. These results are encouraging and warrant further randomised controlled trials, but also vibegron seems not to be a miracle agent showing effects in the range of mirabegron or antimuscarinics. However, there is some light at the end of the tunnel: Closed‐loop optogenetic neuromodulation systems targeting specific neurons to control urinary tract function might completely revolutionise the field, although there are still relevant hurdles to overcome.
Guidelines should result from a rigorous and transparent process informed by the best available up‐to‐date evidence and safeguarded against biases and conflict of interests. This is a major challenge, and from a bird’s eye view, it is hard to comprehend that several guidelines on the same topic exist and it is even more difficult to understand that the recommendations of these guidelines are not congruent and sometimes even contradictory. For instance, in the case of a pelvic organ prolapse repair in a continent woman, the ACOG / AUGS, AUA / SUFU, and EAU guidelines discuss prophylactic anti‐incontinence surgery as an option, whereas ICI and NICE guidelines explicitly recommend against it. The redundancy is enormous, but societies and organisations still create their own guidelines – an unnecessary waste of resources. In recent times, the coronavirus pandemic has rapidly changed our life and paralysed our usual activities, we have to stand together, it is definitively time to join forces! The relevant societies and organisations should consult each other and coordinate their efforts. Together we are strong, let’s move forward to joint guidelines!