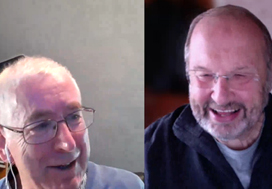
Podcast from BJUI Knowledge: professionalism for urologists
Kieran O’Flynn and Steve Payne discuss professionalism in urology.
BJUI Knowledge: The CPD portal for urologists
Comprehensive, trustworthy and easy to use e-learning platform for trainees, residents, consultants and all specialising in urology.
- Accredited by the Royal College of Surgeons Edinburgh and the College of Surgeons of Hong Kong.
- Approved by the Royal Australasian College of Surgeons.
BJUI Knowledge is evidence-based, fully referenced and peer reviewed to ensure academic, scientific and editorial validity.